Cellulose dissolution and Schweizer's Reagent
Hi all,
I just received an order of 1 lbs of Copper (2) Sulphate pentahydrate ordered from amazon. I will be attempting to produce schweizer's reagent using the copper sulphate for the purpose of dissolving and precipitating cellulose. One roadblock I've come across is obtaining ammonium hydroxide of a substantially high concentration. After finding the msds of the ammonia I have and browsing some home chemical forums, I found that it only has a concentration of around 3% which would make my reagent very dilute.
The actual process for the creation of schweizer's reagent is coming from the youtuber Nile Red who makes many fun and informative videos on extractions, and different organic syntheses. The procedure is as follows, precipitate copper hydroxide by mixing a solution of the copper sulphate and sodium hydroxide, then decant off the remaining solution of sodium sulphate and rinse with fresh water. Then mix in the ammonium hydroxide solution to create a deep blue/purple solution of schweizer's reagent.
The reagent itself is a complex of copper hydroxide and ammonia that is denoted as tetraamminediaquacopper dihydroxide and has the formula [Cu(NH3)4(H2O)2](OH)2.
Here's a model of it that I found on Wikipedia.
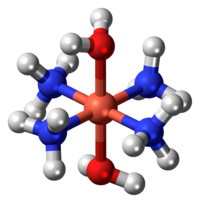
As far as the dissolution of cellulose goes, schweizer's reagent is one of many ways to dissolve it. After browsing an ACS review of the different methods found here, I learned that schweizer's reagent is one of many transition metal complexes that can deprotonate hydroxyl groups of the cellulose, and thereby disperse the molecular structure.
I just received an order of 1 lbs of Copper (2) Sulphate pentahydrate ordered from amazon. I will be attempting to produce schweizer's reagent using the copper sulphate for the purpose of dissolving and precipitating cellulose. One roadblock I've come across is obtaining ammonium hydroxide of a substantially high concentration. After finding the msds of the ammonia I have and browsing some home chemical forums, I found that it only has a concentration of around 3% which would make my reagent very dilute.
The actual process for the creation of schweizer's reagent is coming from the youtuber Nile Red who makes many fun and informative videos on extractions, and different organic syntheses. The procedure is as follows, precipitate copper hydroxide by mixing a solution of the copper sulphate and sodium hydroxide, then decant off the remaining solution of sodium sulphate and rinse with fresh water. Then mix in the ammonium hydroxide solution to create a deep blue/purple solution of schweizer's reagent.
The reagent itself is a complex of copper hydroxide and ammonia that is denoted as tetraamminediaquacopper dihydroxide and has the formula [Cu(NH3)4(H2O)2](OH)2.
Here's a model of it that I found on Wikipedia.

As far as the dissolution of cellulose goes, schweizer's reagent is one of many ways to dissolve it. After browsing an ACS review of the different methods found here, I learned that schweizer's reagent is one of many transition metal complexes that can deprotonate hydroxyl groups of the cellulose, and thereby disperse the molecular structure.


Comments
Post a Comment